FROM POTENTIAL TO POSSIBILITIES
Claris has been recognised for its conducive work environment, value-based & friendly work culture, people-oriented work practices, culture of celebrations, and development orientation.
An erstwhile pharma major, Claris has now pivoted itself for new growth and opportunity through diverse business interests.
Claris Ltd. (Claris) is a holding company established with the long-term objective of diversifying into multiple businesses driven by the spirit of innovation, and a desire to make sustainable impact. Powered by our experience in building large scale global businesses from scratch, efficiently and quickly, Claris has positioned itself for new growth and opportunity through investments and management interest in the fields of clean energy, venture capital and incubators.
Our genesis dates back to 1999, when we set up Claris Lifesciences, a healthcare company which specialised in niche and high-entry barrier businesses with a focus on specialty injectables and infusions.
At its peak, the infusions business, which was India and emerging markets centric, had more than 50% market share in the IV fluids segment, in the regions and product SKUs where we were present. In the injectables space, Claris was one of the largest pure play sterile generic injectables companies in India, with approvals from some of the most stringent regulatory bodies in the world, such as the US FDA, MHRA (UK), and TGA (Australia), among others.
In the span of two decades, the company created nearly a billion-dollar valuation, established a world-class integrated business model, introduced over 130 products, built presence in over a 100 countries, had a team of over 4,000 staffers & workers, and was also listed on the Bombay Stock Exchange from 2010 until 2018.
The years 2017-2018 saw the exit of Claris from healthcare. The injectables business was sold to Baxter Inc., USA for a consideration of $625m (Rs4237cr) after delivering a year-on-year growth of 48% from 2013 until 2017. The infusions business, which was valued at $250m (Rs1300cr) in 2013 at the time of entering a JV with Otsuka Pharmaceutical Factory, Inc., Japan and Mitsui & Co. Ltd., Japan where they were given an 80% stake, was completely sold to Otsuka in 2017. The company also after having successfully raised Rs300cr through an IPO listing in 2010, voluntarily delisted from the BSE in 2018, with a payback of over Rs1073cr to investors.
Claris sets a classic example of a business that successfully transitioned from being family-centric to a professionally-run enterprise. At the helm since 2008, Vice Chairman and MD Arjun Handa brought accelerated growth that saw Claris leverage cutting-edge manufacturing capabilities. A former national-level football player, Arjun credits the game for driving his vision to target difficult-to-develop, complex products within the injectables space, which is characterised by high-entry barriers. The lessons he learnt on the pitch, from team spirit to perseverance, have reflected in his ability to strategise, inspire and lead.
Arjun’s commitment to a people-centric culture has helped Claris win The Economic Times and Great Place to Work® Institute, India’s ‘India’s Best Companies To Work For’ award, 8 years in a row. Other accolades included Fortune India’s ‘The Next 500’, Business Standard’s ‘BS 1000’ and Inc. India’s ‘Innovative 100 List’. Arjun has also been recognised for his entrepreneurial and leadership qualities by The Economic Times (Promising Entrepreneurs of India Award) and Enterprise Asia (Asia Pacific Entrepreneurship Award in the Most Promising category), amongst others.
Parallel to striving for excellence, Claris also serves as a socially responsible organisation, fulfilling goals beyond business commitments. We support and encourage various social causes and focus our capabilities on creating opportunities in the sports and fitness sector, building ecosystems for business and entrepreneurship, and turning the spotlight on art and culture.
‘Claris Lifesciences Limited’ is incorporated as a pharma trading company dealing in Blood Products.
More
Targets international markets through niche and difficult-to-manufacture drugs; inauguration of first injectables manufacturing facility.

Foundation of Active Pharmaceutical Ingredient (API) manufacturing facility; enters the infusions business with a focus on India and other emerging markets; receives the ISO 9001:2000 certification.

Foundation of second infusions manufacturing facility Clarion IV; receives the MHRA (UK Regulatory Approval) for the injectables plant and its first product approval.

Carlyle Group invests $20m (Rs90cr) for a minority stake in the business; receives the European approval for Propofol in LCT form in 11 countries.

Receives the US Food & Drug Administration (FDA) approval for the injectables manufacturing plant; reaches Rs100cr EBITDA at a consolidated level.

Our products now serve 76 countries; flagship product Profol/Provive becomes the #1 propofol brand in India with a 55% market share.

Strategic partnership with Pfizer to target the USA, Canada, Europe, Australia and New Zealand markets; wins awards for quality and manufacturing excellence.

Debut at the BSE with an IPO of Rs300cr, diluting 20% of the Company; wins the ‘India’s Best Companies To Work For’ award, ranking #1 in ‘Healthcare’ and #37 among ‘The 100 Best Indian Companies to Work For’.

Launch of ‘Claris Otsuka’, a joint venture with Otsuka Pharmaceutical Factory, Inc. and Mitsui & Co. Ltd., valued at $250m (Rs1300cr).

Launch of second injectables manufacturing facility Clarion V; focus on the newer technologies of aseptic filling.

One of the lowest attrition rates in the Pharma industry at 15% versus the industry average of 25%; wins the ‘India’s Best Companies To Work For’ award for the seventh year in a row.
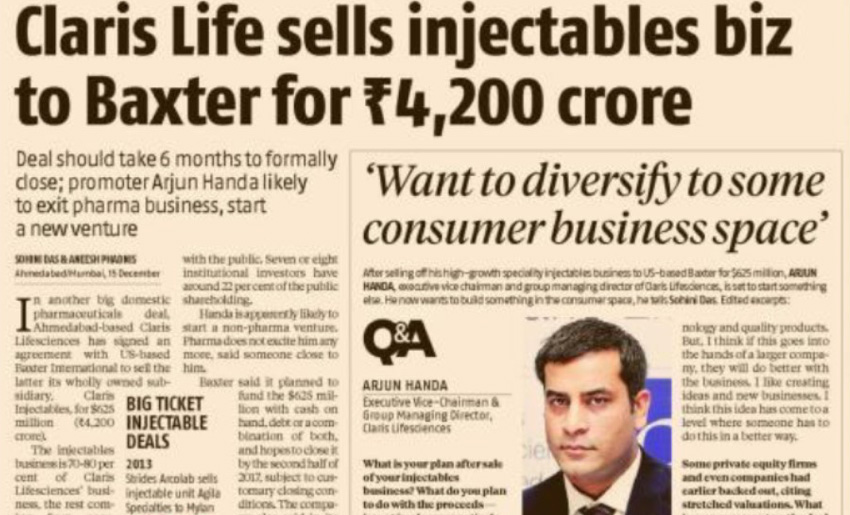
Hits 48% growth for a consecutive fourth year for the specialty injectables business; now present in over 100 countries; global generic Injectables business is sold to Baxter for $625m (Rs4237cr).

Voluntarily de-lists from the Bombay Stock Exchange; returns Rs1,073cr to shareholders as part of the delisting process.

Claris 2.0; focus on venture investment and financial management; enters multiple businesses.
At Claris, you’re recognised for your hard work, you get to work with amazing and talented people, and you can get involved with your community by giving back. It’s the right place for those who seek to build a career for themselves while following their passions.

Claris has been recognised for its conducive work environment, value-based & friendly work culture, people-oriented work practices, culture of celebrations, and development orientation.
Clarion I was the first of five manufacturing facilities set on an 80-acre campus on the outskirts of Ahmedabad. The facility, with box‐in‐a‐box concept, manufactured sterile injectable products. The plant had five separate manufacturing lines: glass ampoules and vials line, large volume parenterals in glass, emulsion manufacturing, non-PVC bags and PVC bags.
Claris' own personal quality standards philosophy, termed ‘Emotional Pharmacopoeia’, was the mantra that guided all manufacturing: it meant that a drug would be considered suitable for sale only if we were comfortable administering it to our loved ones.




We commenced commercial production of sterile infusion products at Clarion II, the second manufacturing facility. This infusion plant was dedicated to blow-fill-seal technology products and was equipped to manufacture products in various volumes in the IV fluids and antibiotic segments. The API plant, Clarion III, was dedicated to manufacturing highly complex and difficult-to-source APIs meant solely for captive consumption. Our manufacturing facility received the ISO 9001:2000 certification for quality management systems.




Clarion IV had the same manufacturing lines as Clarion I, for large volume parenterals in glass, emulsion manufacturing and non-PVC bags, but housed more sophisticated and advanced equipment, achieving higher manufacturing capacities, including a fully automatic bag manufacturing machine which was used to produce single, double and triple chamber bags.




Global private equity firm The Carlyle Group’s investment helped us expand existing capacity and funded R&D and product development initiatives.
Claris proficiency in lipid-based technology made it one of the few in the world to manufacture propofol, a flagship product in anaesthesia. Propofol is an injectable anaesthetic drug that allows targeted use of the drug, for even extremely short durations, i.e., ten minutes, without any side effects, and is a technically-difficult product to manufacture. We also developed a value-added variant, Propofol MCT-LCT for the European market.




The Claris injectables facility received US FDA approval. The US FDA has the world’s most stringent approval standards; we won our first Gold Award at the prestigious India Manufacturing Excellence Awards from Frost & Sullivan




Our presence across regulated and emerging markets stood at 76 countries.
Our performance eventually won us our second Gold Award at the prestigious India Manufacturing Excellence Awards from Frost & Sullivan.




Claris entered into a strategic partnership with global pharma giant Pfizer, for marketing and supply of 15 Claris-manufactured sterile injectables as well as to develop 48 products for the highly regulated markets of the USA, Canada, Europe, Australia and New Zealand. Between 2006 and 2009, Claris had more than 50% market share in the IV Fluids business, in the regions and product SKUs where we were present.
We won our third Gold Award in the India Manufacturing Excellence Awards, as well as the Indian Drug Manufacturers' Association Quality Excellence Award.





We successfully debuted at The Bombay Stock Exchange with an IPO of Rs300cr., diluting 20% of the Company; we won the prestigious ‘Great Place to Work’ award, ranking #1 in ‘Healthcare’ and #37 among 100 of ‘India’s Best Companies To Work For’.




‘Claris Otsuka’ was formed after giving Japan-based companies Otsuka Pharmaceutical Factory, Inc. (one of the biggest Japanese pharmaceutical companies) and Mitsui & Co. Ltd. (one of the most diversified and comprehensive trading, investment and service enterprises in the world), 80% stake in the infusions business. The deal was at a valuation of 3.7x the turnover vs. the industry standard of maximum valuation at 2x the turnover. The funds raised went towards debt prepayment, buyback of shares and general corporate purposes. At this point, Claris was the only company in India to use blow-fill-seal and closed infusion delivery system technologies in manufacturing.
We won our fourth ‘India’s Best Companies To Work For’ award, ranked #1 in ‘Healthcare’ for the fourth time in a row, and #15 among ‘Top 100 India’s Best Companies To Work For’ by The Economic Times and Great Place to Work® Institute, India.

.jpg)


The Clarion V injectables manufacturing facility was in line with the growth strategy and 'injectables focus' that manufactured products on newer technologies.




Claris had the lowest attrition rate in the Pharma industry at 15% versus the industry average of over 25%; we won ‘India’s Best Companies To Work For’ award for the seventh year in a row.

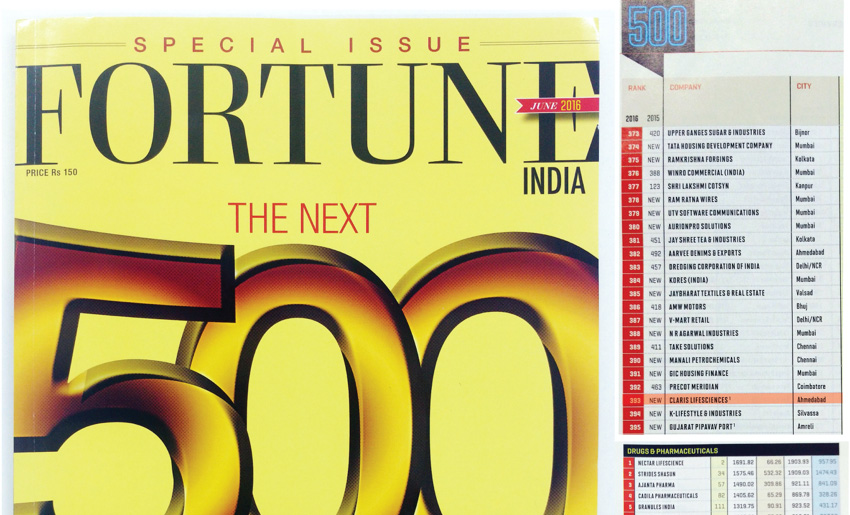


After a consistent growth of 48% for four years, for the specialty injectables business, and a presence in over 100 countries, the global generic injectables business was sold to Baxter International Inc., USA (a Fortune 500 American Healthcare company) and all existing debt of the company was repaid. We also completed the sale of our stake in ‘Claris Otsuka’ and exited from the Joint Venture.
Our winning streak continued with the ’India’s Best Companies To Work For’ award for the eighth year in a row and we won a spot on the coveted ‘The Next 500’ list by Fortune India. Our Vice Chairman and MD Arjun Handa was recognised as one of the ‘The Economic Times Most Promising Business Leaders of Asia 2017-18’ and cited for ‘demonstrating exemplary leadership qualities’.



We voluntarily de-listed equity shares of the company from the Bombay Stock Exchange and returned Rs1,073cr to shareholders as part of the delisting process.






Claris 2.0 is focus on venture investment and financial management, and enters multiple businesses.

.jpg)


Claris has been awarded LEED (Leadership in Energy and Environmental Design) Certificate - Gold Rating for our Corporate Headquarters building, by USGBC (US Green Building Council). The certification was awarded in June 2018, after an assessment of the building. It is even more commendable as in Existing Buildings category, ours is the second building in India to be awarded such Gold certification by USGBC.
LEED is the most widely used green building rating system in the world, providing a framework to create healthy, sustainable, and cost-effective green buildings.

The recognition was conferred in the 2nd edition of ‘The Economic Times Asian Business Leaders Conclave’, which was held at The Majestic Hotel, Kuala Lumpur, Malaysia on November 29-30, 2017.

Claris has been declared as one of ‘India's Best Companies To Work For’ and one of the best companies in ‘Healthcare’ industry in 2017, for the 8th year in a row, by The Economic Times & Great Place to Work® Institute, India.
Our conducive work environment, value-based work culture, and people-oriented work practices were recognised in the study. The awards were received by Shyam Sharma, President - HRM & Corp. Communication and Hardeep Nagar, Sr. GM - HRM & Corp. Communication during the ceremony held at ITC Grand Central, Mumbai on June 30, 2017. The Economic Times too published a special supplement on ‘India's Best Companies To Work For 2017' in its July 3, 2017 edition.
Earlier in the year, we had also been declared as a ‘Great Place to Work® Certified’ organisation by Great Place to Work® Institute, India. It is awarded to the organisations that are rated high in employee responses and people practices.
Over 7,000 organisations across more than 50 countries undergo a Great Place To Work® assessment each year, to measure the level of trust, pride, and camaraderie among their people. The 2017 India study adjudged about 600 organisations, spanning across 20 industries, making it the largest survey of workplace cultures in corporate India.
The consecutive 8th recognition on such a distinguished podium vouches once again that our belief ‘people are everything’ is indeed true to life!

